Product Development
Guest Commentary: Alkermes' four pillars
How Alkermes is structuring development to address complex healthcare environment
For many years, the business of biotechnology was the business of science. Today's complex healthcare environment requires us to think about much more than science to ensure patients gain access to new medicines.
Express Scripts Holding Co.'s agreement with AbbVie Inc. last month on a new HCV treatment made clear this new reality -- one in which a major purchaser of innovative new medicines is willing to negotiate price aggressively and, in so doing, restrict access to competing medicines in a class.
Alkermes plc is transforming the way we evaluate and develop new medicines so that our consideration of this new environment is hardwired into the way we operate. Our job is not simply to develop a medicine, but to develop a substantive and trusted presence in the ecosystems of the disease areas in which we are working.
Our new product development activities are now based on four foundational pillars. The first remains science -- the challenge of capturing new scientific knowledge and insight and translating it into new medicines with new intellectual property. To this we have added three others: economics, policy and people affected.
We chose the four pillars not because they are revolutionary new areas -- we have been working in these areas, at varying levels of intensity, for years. We formalized them in order to bring rigor to the consideration of these areas early and consistently throughout the development process, because these factors are going to have as important an impact on the profile of our medicines as the clinical data supporting their regulatory approvals.
The most immediately obvious difference in applying this framework is the expanded scope of the responsibilities of the project teams, both during development and after new drug approval.
By focusing on all four pillars, new imperatives reveal themselves. Long lead-time issues relating to economic outcomes studies and policy development line up next to carcinogenicity studies and facilities expansion. Our view becomes global to ensure that our new medicines reach the maximum number of patients who stand to benefit from them.
This is a significant project, and it will take some time, but we believe we have the opportunity to create something that can serve as a model for others.
The four pillars
The medicines we choose to develop must have distinctive features in each of the four pillars. Notwithstanding the quality of the science, if a new drug does not differentiate across all of these domains, it is a cause for concern and forces an assessment of the obstacles that may impede its ultimate use. We define these pillars as follows:
Science: A differentiated mechanism of action or other property of the new medicine (perhaps relating to its safety, efficacy or ease of use) that is new, patentable and relevant to patients, healthcare providers and payers, making the medicine unique and, ideally, non-substitutable.
Economics: The economic justification for the use of the medicine in the real world versus competing approaches to treating the same disease. Truly valuable new medicines will disrupt an existing equilibrium in a market. They can affect the economics of payers, providers and patients, as well as those of larger economic units like healthcare systems, counties, states and countries. We need to quantify the effects of this disruption.
Policy: Our medicines are going to be used in large, complex, bureaucratic systems where critical decisions are often made by individuals other than healthcare professionals. We need to understand, and seek to shape over time, the policy framework affecting the use of our medicines in government systems -- both federal and state -- and commercial systems.
People Affected: The collaborative role patients, their families and their communities will play in the conceptualization, development and evaluation of the value of new medicines, and the central role these groups have in assuring access to them. We focus on understanding key relationships that we need to build and the proper methods we need to employ to engage with patients, families and their advocacy communities.
New framework
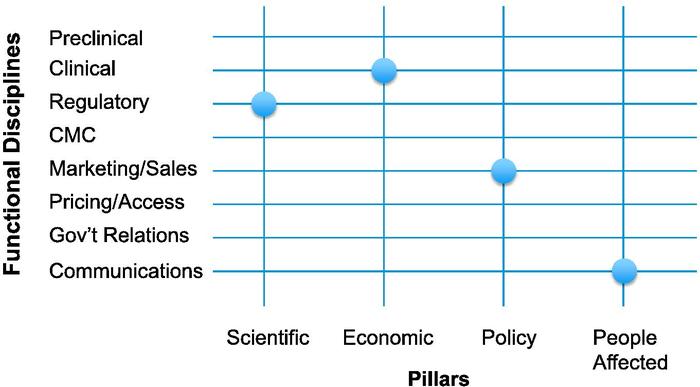
Introducing the imperatives of the four pillars across a representative sample of disciplines that collaborate in the development of a new medicine creates a framework in which each intersection, or node, has potential meaning (see "Framing R&D Decisions," page 16).
We look at each of the nodes sequentially and ask the questions raised by the intersection: First, how does our commitment to that specific pillar affect how we will deploy our resources in that specific discipline? Then, how can we deploy that specific functional discipline in a new way to reinforce the fundamental attributes of the medicine represented by that specific pillar?
While we consider all of the nodes in the matrix for each of our development candidates, certain key nodes clearly emerge. Each program ends up having its own fingerprint -- the particular array of nodes highlighting the need for the most work, the longest lead times, or the most innovative thinking. This tells us where we should direct resources and affects our budgets and our staffing decisions.
This is best demonstrated by looking at four examples drawn from our experience with ALKS 5461, a new medicine to treat major depressive disorder currently in Phase III trials. ALKS 5461 interacts with the brain's opioid system and represents a potential new class of depression medicines with a distinct mechanism of action.
Science and regulation
The questions at the intersection of the scientific pillar and the regulatory discipline are among the most familiar ones, as this intersection has always been at the core of new drug development. However, in addition to asking what regulatory strategy would lead to timely approval with product labeling that allows us to describe ALKS 5461 as a new class of antidepressant with a distinct MOA, we also ask the converse. Namely, how should we structure interactions with regulators over time to build their understanding of how ALKS 5461 differs from other medicines they have reviewed and bring it as efficiently as possible to the patients who need its particular attributes?
In answering the first question, we decided that an essential element of our U.S. regulatory strategy should be to seek Fast Track designation from FDA to increase our opportunities for scientific interaction with the reviewing division.
We recognized that a new MOA was only meaningful if it translated into new benefits for patients. This meant we need to establish that ALKS 5461 can provide clear clinical benefit for patients who are not achieving adequate clinical relief with standard therapies.
In addition, we need to provide data supporting two distinctive features of ALKS 5461: first, its ability to achieve antidepressive effects rapidly; and second, that we have successfully removed the addictive properties of an opioid while preserving its effects on mood.
Answering the second question forced us to think about the development program over time and how we were going to use our opportunities for interaction with regulators most effectively.
These interactions should focus on both conveying phase-appropriate data and answering key development questions, and on contextualizing the evolving data set so that, in the end, the benefit/risk determination can be made with a full understanding of how ALKS 5461's characteristics augment and expand the treatment armamentarium.
This may require developing new approaches to capturing patients' experiences with current treatments and determining the most appropriate way to provide these data to regulators during development and review.
Economics and the clinic
At the intersection of the economic pillar with the clinical function, the first key question was what is the critical distinctive economic feature of ALKS 5461 that will allow us to make a compelling case for its use, knowing that payers will have huge incentives to utilize only generics?
The answer led to one of the most profound decisions in the program: to develop ALKS 5461 as an adjunctive treatment, along with first-line treatment, for patients failing to achieve adequate clinical response. In this way, we would focus development on a subset of patients for whom inexpensive generics were clearly not working, and the next step would involve more expensive and less well tolerated alternatives. We are conceding the point that payers will choose generic alternatives at the outset.
This decision had critical implications. First, it made demonstrating efficacy in clinical trials more difficult because patients in our placebo-controlled studies would still be receiving active therapy rather than placebo alone. Second, it limited our target patient population to only patients who failed on other therapies.
Despite the added complexity, this was attractive because we recognized that these were precisely the patients for whom there was general alignment among patients, physicians, regulators and payers as to the need for new treatment alternatives, and that this subset included millions of patients in the U.S.
The second question is one all biopharmaceutical companies should be asking today: How can we use the resources available to our clinical research team over the course of the clinical program to bolster the value argument?
Answering it leads to the incorporation of a range of additional secondary endpoints in conventional efficacy studies and the development and testing of new exploratory endpoints. It leads also to considering a separate cohort of studies focused primarily on economic outcomes and sequenced over the expected patent life of the product.
Policy and the marketplace
In examining the intersection of the policy pillar with the sales and marketing function, our first question was, do ALKS 5461's unique characteristics create the opportunity for systems to benefit by adapting policies to accommodate its use, or, alternatively, can we ensure that ill-conceived policies do not impede its being available for the patients for whom it is best suited?
We recognized that, with time and the right data, we could potentially help to shape the policy environment for a new medicine to treat refractory depression for the benefit of patients and payers. As the Affordable Care Act is implemented across the country, government and commercial formularies are making decisions about the availability of specific medicines in each class. Depression medicines are currently one of the "six protected classes" under Medicare Part D, and the Mental Health Parity Act guarantees certain benefits for patients with mental illnesses.
Any changes in these policies could have potential positive or negative impacts on our ability to bring ALKS 5461 to patients. For this reason, we are building state and federal policy teams to work with policy makers on the implementation and evolution of these and other policy matters.
The more provocative question was: If policies adapt to accommodate the use of ALKS 5461, can we consider non-traditional commercial structures?
This question challenges the current pharmaceutical commercial model, which relies on field-based salespeople calling primarily on physicians and treatment centers. If policies regarding treatment algorithms are adopted and lead to very clear utilization of ALKS 5461 in specific patient populations by large treatment systems, are conventionally configured field forces the most effective means to disseminate information? This is a work-in-progress and the answers are still being debated.
People and communication
At the intersection of the people affected pillar with the communications function, the first key question was, if our goal is to make a medicine that has a profound impact on patients' quality of life, how should we capture their experiences to provide an accurate depiction of ALKS 5461?
The answer has led us to begin to build a new function based entirely on a deep commitment to this pillar. We see this as going well beyond what is typically described as a "patient advocacy" role within a biopharmaceutical company because it extends beyond the patient to that patient's direct caregiver, family, and in some cases, community.
We want to bring the voice and perspective of these groups to the table throughout the discovery, development and commercial phases of our programs. This requires developing new methods to gain consistent access to their insight, in addition to working with established patient advocacy groups. We cannot wait until late in the program to begin to understand the real-world experience of the people affected, directly or indirectly, by the use of our medicine.
Looking at the node from the other direction led us to ask how can our communications team build a program to ensure that all the people affected by major depression understand that ALKS 5461 and opioid modulation may be a suitable treatment alternative?
This question requires great care and collaboration with our communications, legal and regulatory teams. With that said, it is an essential part of our job -- to determine the most effective ways to convey honest information about our medicines to the people who stand to benefit from them most. We are considering the next generation of tools, beyond standard pharmaceutical direct-to-consumer advertising and traditional unbranded "disease awareness" campaigns.
Many models
There are many different methods that could be employed by companies seeking to introduce important new inputs into the decision-making processes supporting pharmaceutical R&D. At Alkermes, we are working through the development of this new system to drive the consideration of factors outside of those that traditionally dictated the architecture of our development programs.
If we are successful in integrating this expanded sensibility into all of our development programs, we improve the chances that we can make good on the promise of our science and give life to important new medicines welcomed and valued by patients, their caregivers and families, and other critical stakeholders in the increasingly complex healthcare ecosystem.
Companies and Institutions Mentioned
AbbVie Inc. (NYSE:ABBV), Chicago, Ill.
Alkermes plc (NASDAQ:ALKS), Dublin, Ireland
Express Scripts Holding Co. (NASDAQ:ESRX) St. Louis, Mo.
U.S. Food and Drug Administration (FDA), Silver Spring, Md.
Figures
Framing R&D Decisions
To ensure patients gain access to new medicines, Alkermes plc (NASDAQ:ALKS) product teams are incorporating an assessment of what it calls the four pillars: science, economics, policy and people affected. The company applies the framework below to each development program by looking at each of the nodes where the pillars intersect with functional disciplines, and asking the following questions: First, how does our commitment to that specific pillar affect how we will deploy our resources in that specific discipline? Then, how can we deploy that specific functional discipline in a new way to reinforce the fundamental attributes of the medicine represented by that specific pillar? Source: Alkermes plc